What are Alkenes?
Alkene Facts And Things You Should Know!
Alkenes are very useful to us in our daily lives, and they appear much more than you think. Learning about them isn't hard if you know these basic facts below. Of course it doesn't explain how to draw them, but that will be explained further down the article! So what do I positively need to know?
- Alkenes contain contain at least one double bond between the carbon atoms.
- Alkenes are unsaturated, as they have spare bonds that they can use. This is especially useful in making polymers.
- Alkenes decolourise bromine water.
- Alkenes form polymers. This is because their double bonds open up to join other monomers, but this will be explained in more detail further down the article.
- Alkenes burn with a smoky flame. This means that is incomplete combustion, where not enough oxygen is getting to the burning object, which means that it produces carbon dioxide, carbon monoxide, water and carbon (soot).
- The general formula for alkenes is CnH2n. This formula will make sense in a minute!
Alkenes General Formula
The general formula for alkenes is CnH2n. Unlike alkanes, alkenes have a simpler formula. The "C" stands for carbon and the "H" stands for hydrogen, that is why alkenes come from the hydrocarbon family. The "n" part stands for any number, which remains the same for both "n" parts. All that this formula is showing us, is how many carbon atoms there are and how many hydrogen atoms there are in a certain alkenes. Also the "2" after the "H" means times "n" by two. Here is an example if you are not sure:
Example:
Let's take propene, it has three carbon atoms. So to work out the hydrogen atoms, we have to use the formula CnH2n. Input the number three where the "n" is in the formula. Now we have a sum to do - two multiplied by three. This gives us six hydrogen atoms. Straightforward isn't it?
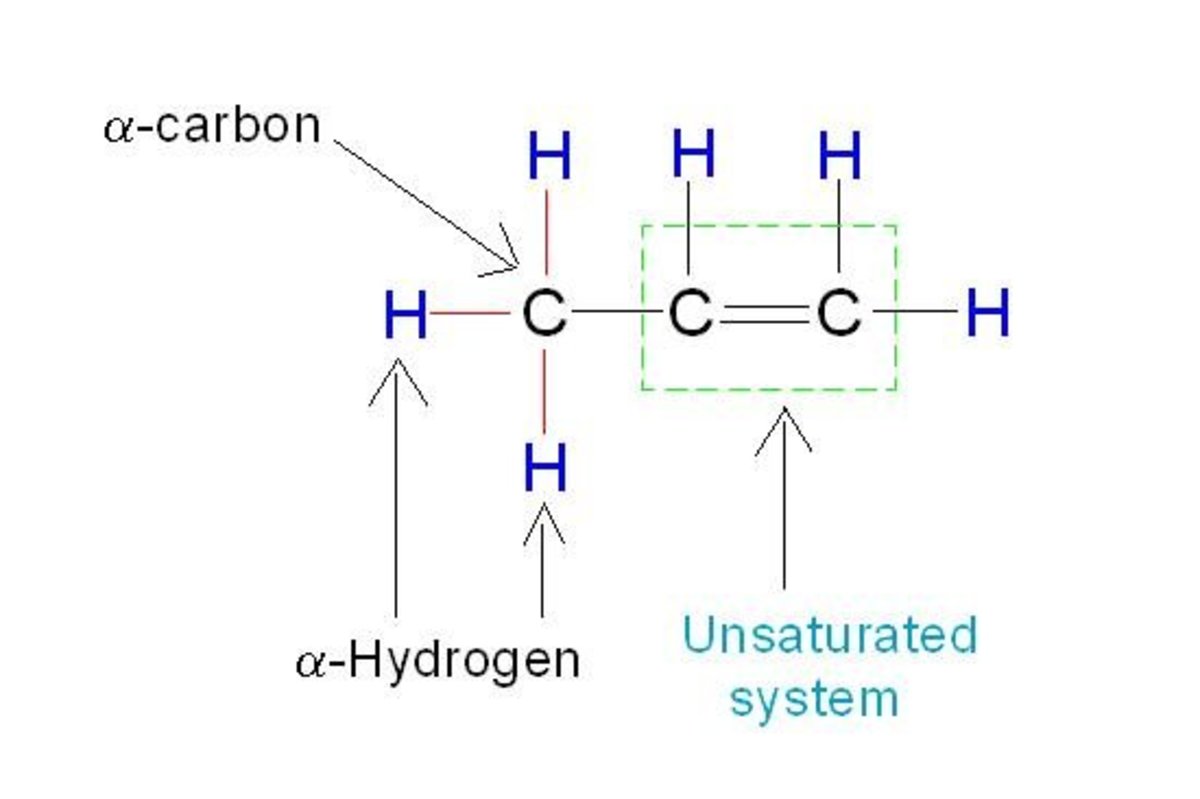
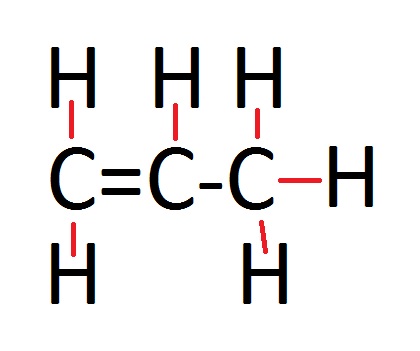
Displayed Formula
If you are ever lucky enough to receive a question where they want the displayed formula, then all that means is that you have some drawing to do! Showing the displayed formula is just a little diagram, which is very effortless to do. The picture adjacent to the general formula shows you how you show the displayed formula. All you have to remember, is that alkenes contain a double bond. So from one carbon to carbon you are going to have to draw two lines to represent the bonds, and the rest of the bonds from hydrogen to carbon and carbon to carbon you only need to draw one bond (which wil be shown by one single line).
In the example photo, it is propene which has the formula C3H6. This shows what I have been talking about, it has only one double bond and the others are all single bonds. Simply draw three carbon atoms with a double bond linking two of them and then two hydrogen atoms coming off the double bonded carbon atom. Then add one hydrogen atom to the other carbon atom that is linked by the double bond and the remainding carbon atom will have three hydrogen atoms bonded to it.
Have you been learning?
How many hydrogen atoms to carbon atoms are there in alkenes?
What are alkenes used for?
Alkenes have one major use that we see all the time in our daily lives. That is plastic! Alkenes form monomers and then join together to form polymers to make plastic. Polymers are made by the double bonds in the alkene breaking open. This spare bond then grabs another carbon atom and then this happens millions of times with millions of different monomers to form a polymer. Some polymers you might have heard of are polystyrene, polyethylene, polythene and polypropylene.
Alkenes are also used for fuels and alkenes were also used in mustard gas that was used in world war two. Also alkenes can be used in food production to ripen up fruits.
Alkenes: Their Formula And Boiling Point
Name Of Alkene
| General Formula
| Boiling Point
|
|---|---|---|
Butene
| C2H4
| -6.6 °C
|
Propene
| C3H6
| -47.6 °C
|
Ethene
| C4H8
| -103.7 °C
|
Pentene
| C5H10
| 30 °C
|
Hexene
| C6H12
| 63 °C
|
Heptene
| C7H14
| 94 °C
|
Octene
| C8H16
| 121 °C
|
What an alkene is really like. The displayed formula is much easier to understand.
Breaking alkenes down
Breaking alkenes down have many advantages. You can turn large chain alkenes that are not very useful into smaller chain alkenes that can be used to make plastic or be used as a fuel. The process is often referred to as cracking in industry. So what is cracking?
Cracking is simply breaking down large hydrocarbon chains into shorter hydrocarbon chains, so we they are more useful to us. There are two ways you can crack a hydrocarbon. First of all you have to make the hydrocarbon into a vapour. Then you can either:
- Pass it over a hot catalyst.
- Mix it with steam and heat it to a very high temperature.
The process breaks chemical bonds in the molecules, causing thermal decomposition to happen which breaks apart the molecules.
How much have you learnt?
view quiz statistics
That's the basics
You have now learnt the basics. You can now claim that you are a scientist! Have you tried the quiz yet? If you have any more questions about alkenes, then leave a comment and I will get back to you ASAP.
© 2014 crazyduck